PROTECT YOUR DNA WITH QUANTUM TECHNOLOGY
Orgo-Life the new way to the future Advertising by Adpathway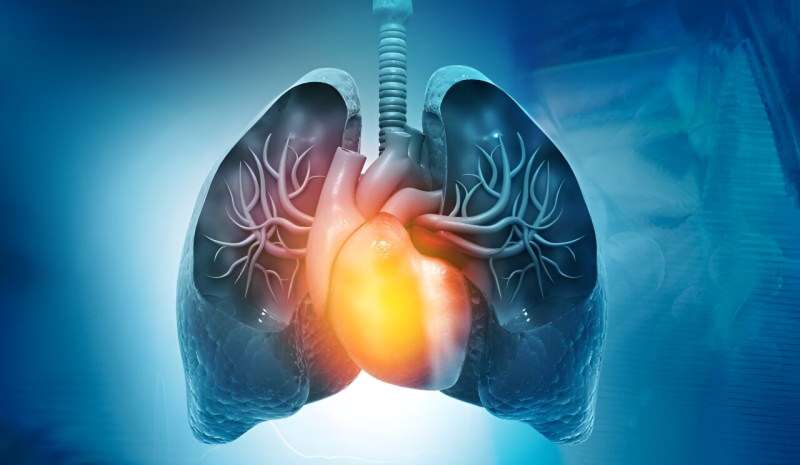
The U.S. Food and Drug Administration has approved Winrevair (sotatercept-csrk) as an injectable treatment for pulmonary arterial hypertension (PAH) in adults.
The FDA previously granted Winrevair a breakthrough therapy designation. It is the first FDA-approved activin signaling inhibitor therapy for PAH, representing a new class of therapy. Winrevair works by improving the balance between pro- and antiproliferative signaling to regulate the vascular cell proliferation underlying PAH.
The approval was based upon data from the Phase III STELLAR trial, which compared Winrevair (163 patients) to placebo (160 patients) in adult patients with PAH. Findings show that adding Winrevair to background standard-of-care therapy increased six-minute walk distance from baseline to week 24 by 41 meters.
Significant improvements were also seen in the secondary outcome measures of risk of death from any cause and PAH clinical worsening events by 84 percent (number of events, nine versus 42; hazard ratio, 0.16).
"New treatment options continue to be needed for patients with pulmonary arterial hypertension that support important clinical goals, including increasing exercise capacity and improving functional class," STELLAR study investigator Aaron Waxman, M.D., of Brigham and Women's Hospital in Boston, said in a statement.
"Sotatercept added to background therapy has the potential to become a new standard-of-care option for patients with pulmonary arterial hypertension."
More information: Merck News Release
© 2024 HealthDay. All rights reserved.
Citation: FDA approves Winrevair for pulmonary arterial hypertension in adults (2024, March 29) retrieved 29 March 2024 from https://medicalxpress.com/news/2024-03-fda-winrevair-pulmonary-arterial-hypertension.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.


 1 month ago
17
1 month ago
17
















.jpg)






 English (US) ·
English (US) ·  French (CA) ·
French (CA) ·